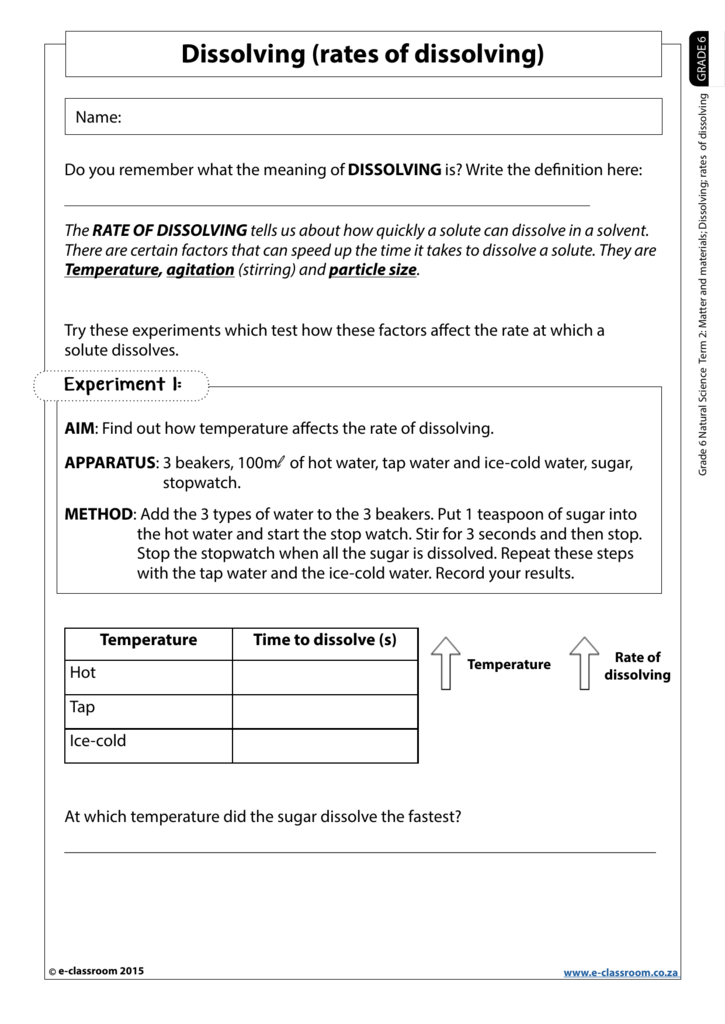
How Does Temperature Affect the Rate of Dissolving
Insolubility is the opposite property the inability of the solute to form such a solution. Moving out from there the temperature increases until you reach the surface where the ice has warmed to a temperature of pu0 circ C and has begun melting.
It is relayed to the central nervous system rapidly by A delta nerve fibers.
. The surface layer of. In chemistry solubility is the ability of a substance the solute to form a solution with another substance the solvent. Pain that typically is produced by sudden injury eg fracture or illness eg acute infection and is accompanied by physical signs such as increased heart rate elevated blood pressure pupillary dilation sweating or hyperventilation.
Now we can readily see why adding salt to the liquid surrounding the ice chunk would cause a lowering of the temperature of that salty melt-water. A variation of just a few degrees from this temperature alters the activity of the microbes and affects the quality of the final product. By adding salt you have lowered the melting point.
Temperature Different yeasts tolerate different temperatures. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution one in which no more solute can. Then at the end of this entry.
For Saccharomyces cerevisiae it is around 35-400C. Acute pain is typically sharp in character. The rate of fermentation can be controlled by manipulating any of these factors.

Investigating The Factors Affecting The Rate Of Dissolving Ppt Download

Does Temperature Affect Dissolving Youtube

8 2 Factors That Affect Rate Of Dissolving And Solubility Ppt Download

No comments for "How Does Temperature Affect the Rate of Dissolving"
Post a Comment